- Home
- Products
- Elementary
- Boride Powder
- 3D Printing Powder
- Sulfide Powder
- Oxide Powder
- Carbide powder
- Nitride Powder
- Silicide Powder
- Hydride Powder
- Telluride Powder
- Selenide Powder
- Stearic Acid Series
- Phosphide Powder
- Nanoparticles
- Metal Alloy
- MAX Phase
- Lithium Battery Anode
- Surfactant
- Molecular sieves
- Concrete Admixtures
- News
- Answers
- Contact
- About
News
- 1
- 1
Eight properties of molybdenum disulfide revealed: a dark horse in the field of lubricating materials
If you are looking for high-quality products, please feel free to contact us and send an inquiry, email: brad@ihpa.net
1. The relationship between friction coefficient and industrial and mining conditions:
The friction coefficient of graphite is very low, generally 0.03-0.09 in the atmosphere, the minimum is 0.006-0.03, and the maximum is 0.15-0.25, which is lower than graphite. In vacuum, the minimum is 0.001, and the maximum is 0.2, that is, it has better performance in vacuum. lubricity. And it decreases with the increase of temperature and load, and decreases with the increase of sliding speed, and can reach a minimum of less than 0.07. The relative humidity of the air has an impact on the friction coefficient of calcium disulfide. When the relative humidity is lower than 15%, the friction coefficient is low. When the relative humidity reaches 16%, the friction coefficient reaches its peak.
2. Lubricity:
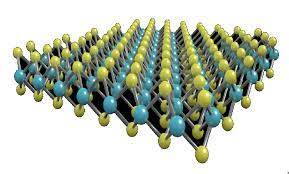
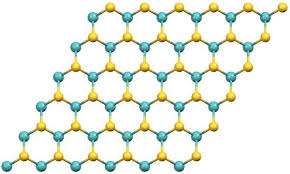
Molybdenum disulfide has a hexagonal crystal layered structure. The crystal structure is composed of three planes: sulfur-molybdenum-sulfur. This structure is like a "sandwich bread", with two sulfur atoms sandwiching a molybdenum atom in the middle. This thin layer of molybdenum disulfide is extremely small. Within the thickness of only 0.25 mm, there are about 40,000 "pieces", that is, molybdenum disulfide monomer molecules, or cleavage planes (or slip planes) or There are 1598 slip surfaces in 0.001 mm (ie = 1/1000 mm = 1 micron), that is, there are 799 slip surfaces in a 0.5 micron thick layer. This is because the strong covalent bond between the sulfur atom and the molybdenum atom in a single molybdenum disulfide molecule in the thin layer is difficult to separate, and there is only a weak van der Waals bond between the sulfur atom and the sulfur atom between the two monomer molybdenum disulfide molecules. The connection (i.e. low adhesion) is easy to split. Therefore, in such an extremely thin layer, for example, there are 799 slip surfaces in a 0.5 micron ultra-thin layer. Such many low shear force and low viscosity slip surfaces make the surface of the two relatively moving objects The friction between the molybdenum disulfide layers is transformed into friction between the molybdenum disulfide layers, which plays a good lubrication effect.
3. Chemical stability:
Molybdenum disulfide has strong corrosion resistance. Except for nitric acid, aqua regia, boiling hydrochloric acid, concentrated sulfuric acid, pure oxygen, fluorine, and chlorine, it has no effect on other acids, alkalis, and drugs. In alkalis with a pH value greater than 10 In neutral aqueous solutions, it can slowly oxidize and is unstable to strong oxidants. It is insoluble in cold, hot and boiling water. It is also stable in petroleum and synthetic lubricants such as mechanical oil and grease, or in eco-friendly solvents such as ethanol and ether: it is stable to surrounding gases. In humid air at room temperature, oxidation is slight, but corrosive acids can be formed. After molybdenum disulfide is added to grease, it is difficult to oxidize without full contact with oxygen in the air, and the wear resistance is improved.

4. Thermal stability:
Molybdenum disulfide has good thermal stability, with a melting point of 1185°C. In the atmosphere, it can ensure good lubrication performance in the range of -184-399℃. Oxidation begins at 400°C, oxidation is significant at 450°C, and oxidation is rapid at 540°C. The final products of oxidation are molybdenum trioxide and sulfur dioxide. While it is not completely transformed into molybdenum trioxide, it still has lubricity. After the formation of molybdenum trioxide, friction coefficient increases. The Mohs hardness of molybdenum trioxide is 2-2.5, and the maximum friction coefficient in the atmosphere is 0.2. Therefore, most people now believe that molybdenum trioxide cannot be an abrasive, because it is also included in the list of 56 solid lubricants. Molybdenum dioxide can be corroded by the alkali metals lithium, sodium, rubidium, calcium, and francium. Molybdenum disulfide is different from graphite when used in vacuum. It has good lubricity and only begins to decompose at 1093°C. This is because when molybdenum disulfide is in the air, the active sulfur atoms on its crystal facets easily interact with it. Oxygen in the air reacts. When it reaches the vacuum, its temperature will be close to the decomposition temperature. It is impossible for removing the oxide layer generated by molybdenum disulfide after adsorbing oxygen. This determines the performance of molybdenum disulfide in vacuum conditions. It can still maintain low bonding between crystals, ensuring low friction performance. Especially under ultra-high vacuum conditions, when the temperature reaches 800°C, the friction coefficient not only does not increase, but decreases. Above 800°C, due to the phase change of the crystal, the lubrication ability gradually decreases. When it reaches 1093°C, it begins to break down. When molybdenum disulfide is transferred from vacuum to atmospheric conditions, the lubrication performance decreases. This is due to the moisture absorption of molybdenum disulfide. This is exactly the opposite of the situation where graphite absorbs moisture and exhibits a reduction in friction coefficient and improvement in lubrication performance. Molybdenum disulfide can still lubricate at low temperatures of -184°C or lower. The friction coefficient can remain unchanged until the temperature reaches -200°C. If the temperature is greater than -200°C, the friction coefficient increases exponentially.
Molybdenum disulfide will remove adsorbed water vapor at high temperatures, which will turn the oxide film on the active surface into a small number of hydroxide compounds. Due to the presence of hydrogen bonds, molybdenum disulfide will form an adhesive film on the metal surface. The adhesion ability decreases, so the ability of molybdenum disulfide to adhere to the metal surface at high temperatures is lower than at normal temperature. Therefore, when using it at high temperatures, we must consider improving the adhesion of molybdenum disulfide to the metal surface. The stronger the adhesion, the better the high-temperature lubrication performance.
5. Extreme pressure resistance:
Molybdenum disulfide has high pressure resistance, which is unequaled by other lubricating materials. It can still be made use of under very high pressures of 20,000 kg/cm2. The film of a general lubricating oil layer has already ruptured under such high pressure, and Loss of lubrication, causing metal surfaces to weld. The test results show that there is molybdenum disulfide between the metal surfaces. When the pressure increases to 32,000kg/cm2, in fact, this pressure has exceeded the yield point of a certain metal, but bite and welding still do not occur between the two metal surfaces. Molybdenum disulfide has been tested on a 2.5 micron film to withstand a contact stress of more than 28.000kg/cm2 and a friction speed of 40 meters/second. This high resistance to pressure is due to its material structure. The compressive strength of graphite is only 200-240000kg/cm2, and that of tungsten disulfide is only 21,000kg/cm2.
6. Adhesion
Molybdenum disulfide has strong adhesion to metals. Its attachment to the metal surface is through the surface of its sulfur atoms and the metal. When sulfur is directly combined with the metal, the binding ability is quite strong and it is not easy to peel off under general friction. However, it is usually not easy to adhere to unclean surfaces, such as surfaces contaminated by impurities, grease, etc. On the other hand, the molybdenum disulfide particle size is extremely fine, so it has a large surface area. The fine particles can easily fill in the uneven valleys on the metal surface. The large molybdenum disulfide contact surface will have a greater chance of contacting the metal. When "sulfur and metal" are in contact, the high heat of friction will cause the convex peaks on the metal surface to flow, causing the molybdenum disulfide molecules to combine with the metal and adhere to the metal surface to form an extremely thin layer of disulfide. The solid layer of molybdenum sulfide is thin. If the surface of molybdenum disulfide in contact with the metal is a smooth surface with high finish, due to the elastic-plastic deformation of the metal, the molybdenum disulfide will penetrate into the metal surface to form a solid film of molybdenum disulfide. This combination and adhesion are It is related to the unevenness of the metal surface, the length of application of molybdenum disulfide, and the high purity and fine particle size of molybdenum disulfide.

7. Conductivity:
The electrical conductivity of molybdenum disulfide is not as good as that of graphite. Its resistivity is 8.51*102 ohms per centimeter, which means it is a poor conductor compared with graphite. However, above normal temperature, as an inorganic substance, it should be said that the conductivity is quite good. But under normal conditions it is a poor conductor and non-magnetic body.
8. Radiation resistance:
Molybdenum disulfide has certain radiation resistance. R-ray radiation increases friction by 50% at room temperature. Neutron radiation does not damage the crystal lattice, so it still has a lubricating effect under the condition of radiation. Experiments have proved: Under the illumination condition of 7*108 Lun, the dynamic and static friction values of molybdenum disulfide and tungsten disulfide remain unchanged, while the dynamic and static friction coefficients of graphite increase greatly, and there are varying degrees of improvement in cloth wear resistance. Decrease, especially graphite has the largest decrease, and tungsten disulfide has the smallest decrease. Molybdenum disulfide is close to tungsten disulfide, that is, the wear amount has increased. The wear amount of graphite is 179.9*10-3 mm3, and the wear amount of molybdenum disulfide is 76.2*10-3 mm3, while tungsten disulfide is 66.4*10-3 mm3.

Supplier
TRUNNANO is a supplier of spherical silica powder materials with over 12 years experience in nano-building energy conservation and nanotechnology development. It accepts payment via Credit Card, T/T, West Union and Paypal. Trunnano will ship the goods to customers overseas through FedEx, DHL, by air, or by sea. If you are looking for high-quality concrete additives, please feel free to contact us and send an inquiry.
Inquiry us
PREVIOUS NEWS
Which is the best way to backfill a bathroom
NEXT NEWS